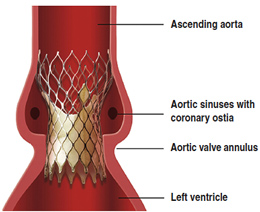
Medtronic, the makers and financial backers of a recent study on the Core Valve used for percutaneous aortic valve replacement (aka non-surgical valve replacement) released their findings showing the six month mortality data on patients receiving this valve.
This study which was performed using data from European cardiologists (who have been using this technology longer) were unsurprising – with a higher risk of stroke and overall mortality. Notably, this study was performed on patients deemed to be ‘at high risk’ for surgery, not ineligible for surgery. As we’ve discussed before, the term ‘high risk’ is open to considerable interpretation.
“ A total of 996 frail, elderly patients at high risk for heart surgery were implanted with Medtronic’s CoreValve device, used to treat severe narrowing of the aortic valve. Mortality rates at one month and six months were 4.5% and 12.8%, respectively. Stroke rates were 2.9% and 3.4%.
Medtronic said the rates were consistent with previously reported data from national registries in Europe.”
Unfortunately, the general media’s coverage of these findings have been less than straightforward as Bloomberg proclaims in blazing headlines, “Edwards heart valve skirts rib-cracking for a 2.5 billion dollar market.” That’s a pretty eye-opening headline that manages to avoid mentioning the real issues – longevity and durability.
Another article from business week proclaims, “Heart Valves found safe.” Safe, I guess is a relative term – if you aren’t one of the 12.8% that died within six months..
More about Aortic Stenosis and Valve Replacement therapies at Cartagena Surgery: (you can also find a link to these stories under the TAVI tab on the sidebar.)
Aortic Stenosis, surgery and the elderly
Aortic Stenosis: New Recommendations for TAVI
Transcatheter Valve Therapies: an overview
Peri-operative outcomes with TAVI