It looks like the rest of the medical community is finally speaking up about the overuse and safety issues of TAVI/ TAVR for aortic stenosis, but it’s still few and far between – and in specialty journals… But in the same week that Medscape, and the Heart.org reported on a newly published article in the British Medical Journal on the overuse of TAVI therapies, and the need for earlier diagnosis and treatment of Aortic Stenosis – the Interventionalists over at the Heart.org (a cardiology specialty journal) have published a series of articles promoting / pushing the procedure including an article entitled, “The TAVR Heart team roles.”
JAMA recently published a paper by Robert Bonow and Chintan Desnai, discussing the benefits, risks and expectations with TAVI. This paper discusses the very real need for clinicians to address heightened patient expectations regarding TAVI as an ‘easy’ alternative to surgery.
TAVI is vastly overused – Reed Miller, The Heart.org
Here at Cartagena Surgery – we’ve been doing our own research – contacting and talking to a multitude of practicing cardiologists and cardiac surgeons to get their opinions – in addition to reviewing the latest data.
In related news, a review of the latest research on the ‘transcatheter’ valve therapies demonstrates considerable concern: including data on peri-valvular leaks as reported in the last national TAVI registries in Europe and in the US:
• The incidence of paravalvular leaks after TAVI is extremely high ( > 60%)
• It is technically challenging today to quantify these leaks.
• Most of them are quoted “mild”, but more than 15 % are estimated “moderate” and “severe”.
• In > 5% of patients, the peri-valvular or valvular regurgitation grade increased significantly over time.
• there is no significant difference between Edwards SAPIEN and Medtronic COREVALVE
As one cardiologist explained:
“Importantly, the thrombogenic potential of mild leaks was recently demonstrated by Larry Scotten ( Vivitro System Inc. Victoria, Canada). High reverse flow velocities expose glycoprotein GP Ib-IX-V platelet receptors to circulating Von Willebrand molecule with, as results, platelet aggregation and fibrin formation. The incidence of brain spots and stroke after TAVI was of great concern in the PARTNER A and B studies. Whereas, Aspirin is not mandatory in patients implanted with bioprosthetic valves, Plavix + Aspirin is recommended for all TAVI patients. The rationales of such therapy were not explained so far.”
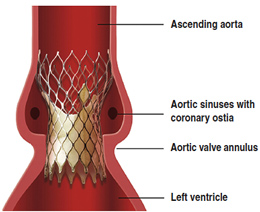
Valve oversizing – a surgeon explains
“To reduce these peri-valvular leaks , cardiologists tentatively use large valve size, up to 29-mm. The very large majority of valve sizes used in conventional aortic valve replacement are smaller than 25-mm. Oversizing may increase the risk of late aortic aneurysms (aortic rupture has been reported) [emphasis added].
Moreover, atrio-ventricular conduction may be impaired with the need of permanent pacing. Poorer outcomes have been reported in patients when the need for permanent pacemaker occurs.
“As we like to say about clothes and shoes, you forget the price overnight but you remember the quality for ever . The price of TAVI may be cheaper but patients may experience inferior outcomes. In view of these results, using TAVI would not be appropriate for the great majority of heart valve candidates. Moreover trans-catheter delivery and sub-optimal fit are not likely to increase tissue valve durability… and everybody knows that tissue valves are not enough durable for young adults and children. TAVI is thus a suitable strategy only for the neglected population of high risk patients who are no longer candidates for surgery [emphasis added].
Worth pointing out again that there would be no need for TAVI and long-term outcomes of patients would be much better if severe aortic stenosis were correctly managed at the right time. Enclosed the recommendations of Robert Bonow (Circulation, July 25, 2012) for early valve replacement in ASYMPTOMATIC patients. A large cohort of accurate biomarkers is available today for correct timing of surgery and consequent prevention of irreversible myocardium damage. In the study of Lancellotti (enclosed) 55% of “truly asymptomatic patients” with severe aortic stenosis developed pulmonary hypertension during exercise and had poor clinical outcomes. The measurement of both mean trans-aortic pressure gradient and systolic pulmonary pressure, which are technically easy, rapid and with good reproducibility may improve the management of such patients.
These updates on the natural history of aortic stenosis illustrate the present paradoxical and intriguing focus of the industry on an experimental procedural innovation for end-stage old patients when more efficient heart valves are today feasible and could be used sooner for the benefit of all patients .
Enclosed an article on The Need For A Global Perspective On Heart Valve from Sir Madgi Yacoub.
Additional Reference / supporting data:
Modified from Ross J and Branwald E (Circulation 1968 (Suppl): 61-67)
• The incidence of stroke was 9% after TAVI in the 214 patients of the enclosed study published last week in the American Journal of Cardiology. The incidence of stroke with TAVI was > two times higher than with conventional surgery in the PARTNER study. Pooled proportion of postoperative stroke was 2.4% with conventional surgery in the large meta-analysis of patients > 80 years old (enclosed)
• Peri-valvular aortic insufficiency is observed in more than 60% of patients undergoing trans-catheter aortic valve replacement. Moderate or severe aortic insufficiency was seen in 17.3 % of the PARTNER inoperable and high risk cohorts at 1 year. They have been reportedly associated with dyspnea, anemia, cardiac failure and diminished survival. Most interestingly, the FDA does not accept more than 1% peri-valvular insufficiency in patients implanted with conventional prosthetic heart valves… The SJM Silzone mechanical heart valve was re-called because of peri-valvular leakage rate of… 1.5 % .
• Traditionally, aortic stenosis involving a 2-cuspid aortic valve has been a contraindication to TAVI. Of 347 octogenarians and 17 nonagenarians explanted valves , 78 (22%) and 3 ( 18%) had stenotic congenitally bicuspid aortic valve, respectively. Because the results of TAVI are less favorable in patients with stenotic congenitally bicuspid valves, proper identification of the underlying aortic valve structure is critical when considering TAVI in older patients . More than 50% of patients with aortic stenosis have bicuspid aortic valve and are not, therefore, good candidates for TAVI. Most importantly, the great majority of patients with calcified stenotic bicuspid aortic valves is young ( < 60 years old) and not candidate for tissue valve replacement.
• The French Registry of trans-catheter aortic-valve implantation in high-risk patients was published in the New England Journal of Medicine on May 3, 2012. It reports 3195 TAVI procedures during the last two years at 34 centers.
The mean age was 83 years. The incidence of stroke was 4.1%. Peri-prosthetic aortic regurgitation was 64 %. The rate of death was 24% at one year. At the same time, the meta-analysis published in the American Heart Journal reports 13,216 CONVENTIONAL AORTIC VALVE REPLACEMENT in patients > 80 years old. The rate of death was 12.4% at one year, 21.3% at 3 years and 34.6% at 5 years
Full references for works cited in text:
Bonow, R. O. (2012). Exercise hemodynamics and risk assessment in asymptomatic aortic stenosis. Circulation 2012, July 25.
Lancelloti, P., Magne, J., Donal, E., O’Connor, K., Dulgheru, R., Rosca, M., & Pierard, L. (2012). Determinants and prognostic significance of exercise pulmonary hypertension in asymptomatic severe aortic stenosis. Circulation, 2012 July 25.
Takkenberg, J. J. M., Rayamannan, N. M., Rosenhek, R., Kumar, A. S., Carapitis, J. R., & Yacoub, M. H. (2008). The need for a global perspective on heart valve disease epidemiology: The SHVG working group on epidemiology of heart disease founding statement. J. Heart Valve Dis. 17 (1); 135 – 139.
Gilard M, Eltchaninoff H, Iung B, Donzeau-Gouge P, Chevreul K, Fajadet J, Leprince P, Leguerrier A, Lievre M, Prat A,Teiger E, Lefevre T, Himbert D, Tchetche D, Carrié D, Albat B, Cribier A, Rioufol G, Sudre A, Blanchard D, Collet F, Dos Santos P, Meneveau N, Tirouvanziam A, Caussin C, Guyon P, Boschat J, Le Breton H, Collart F, Houel R, Delpine S,Souteyrand G, Favereau X, Ohlmann P, Doisy V, Grollier G, Gommeaux A, Claudel JP, Bourlon F, Bertrand B, Van Belle E, Laskar M; FRANCE 2 Investigators. Collaborators (184). Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012 May 3; 366(18):1705-15 [full abstract below].
BACKGROUND:
Transcatheter aortic-valve implantation (TAVI) is an emerging intervention for the treatment of high-risk patients with severe aortic stenosis and coexisting illnesses.
We report the results of a prospective multicenter study of the French national transcatheter aortic-valve implantation registry, FRANCE 2.
METHODS:
All TAVIs performed in France, as listed in the FRANCE 2 registry, were prospectively included in the study. The primary end point was death from any cause.
RESULTS:
A total of 3195 patients were enrolled between January 2010 and October 2011 at 34 centers. The mean (±SD) age was 82.7±7.2 years; 49% of the patients were women.
All patients were highly symptomatic and were at high surgical risk for aortic-valve replacement. Edwards SAPIEN and Medtronic CoreValve devices were implanted in 66.9% and 33.1% of patients, respectively. Approaches were either transarterial (transfemoral, 74.6%; subclavian, 5.8%; and other, 1.8%) or transapical (17.8%).
The procedural success rate was 96.9%. Rates of death at 30 days and 1 year were 9.7% and 24.0%, respectively.
At 1 year, the incidence of stroke was 4.1%, and the incidence of periprosthetic aortic regurgitation was 64.5%.
In a multivariate model, a higher logistic risk score on the European System for Cardiac Operative Risk Evaluation (EuroSCORE), New York Heart Association functional class III or IV symptoms, the use of a transapical TAVI approach, and a higher amount of periprosthetic regurgitation were significantly associated with reduced survival.
CONCLUSIONS:
This prospective registry study reflected real-life TAVI experience in high-risk elderly patients with aortic stenosis, in whom TAVI appeared to be a reasonable option.
Rutger-Jan Nuis, MSc, Nicolas M. Van Mieghem, MD, Carl J. Schultz, MD, PhD, Adriaan Moelker, MD, PhD , Robert M. van der Boon, MSc, Robert Jan van Geuns, MD, PhD, Aad van der Lugt, MD, PhD, Patrick W. Serruys, MD, PhD, Josep Rodés-Cabau, MD, Ron T. van Domburg, PhD, Peter J. Koudstaal, MD, PhD, Peter P. de Jaegere, MD, PhD. Frequency and Causes of Stroke During or After Trans-catheter Aortic Valve Implantation. American Journal of Cardiology Volume 109, Issue 11 , Pages 1637-1643, 1 June 2012 [full abstract provided].
Transcatheter aortic valve implantation (TAVI) is invariably associated with the risk of clinically manifest transient or irreversible neurologic impairment. We sought to investigate the incidence and causes of clinically manifest stroke during TAVI. A total of 214 consecutive patients underwent TAVI with the Medtronic-CoreValve System from November 2005 to September 2011 at our institution. Stroke was defined according to the Valve Academic Research Consortium recommendations. Its cause was established by analyzing the point of onset of symptoms, correlating the symptoms with the computed tomography-detected defects in the brain, and analyzing the presence of potential coexisting causes of stroke, in addition to a multivariate analysis to determine the independent predictors. Stroke occurred in 19 patients (9%) and was major in 10 (5%), minor in 3 (1%), and transient (transient ischemic attack) in 6 (3%). The onset of symptoms was early (≤24 hours) in 8 patients (42%) and delayed (>24 hours) in 11 (58%). Brain computed tomography showed a cortical infarct in 8 patients (42%), a lacunar infarct in 5 (26%), hemorrhage in 1 (5%), and no abnormalities in 5 (26%). Independent determinants of stroke were new-onset atrial fibrillation after TAVI (odds ratio 4.4, 95% confidence interval 1.2 to 15.6), and baseline aortic regurgitation grade III or greater (odds ratio 3.2, 95% confidence interval 1.1 to 9.3).
In conclusion, the incidence of stroke was 9%, of which >1/2 occurred >24 hours after the procedure. New-onset atrial fibrillation was associated with a 4.4-fold increased risk of stroke. In conclusion, these findings indicate that improvements in postoperative care after TAVI are equally, if not more, important for the reduction of peri-procedural stroke than preventive measures during the procedure.
Sinning JM, Hammerstingl C, Vasa-Nicotera M, Adenauer V, Lema Cachiguango SJ, Scheer AC, Hausen S, Sedaghat A, Ghanem A, Müller C, Grube E,Nickenig G, Werner N. (2012). Aortic regurgitation index defines severity of peri-prosthetic regurgitation and predicts outcome in patients after transcatheter aortic valve implantation. J Am Coll Cardiol. 2012 Mar 27;59(13):1134-41. [full abstract provided].
OBJECTIVES:
The aim of this study was to provide a simple, reproducible, and point-of-care assessment of peri-prosthetic aortic regurgitation (periAR) during trans-catheter aortic valve implantation (TAVI) and to decipher the impact of this peri-procedural parameter on outcome.
BACKGROUND:
Because periAR after TAVI might be associated with adverse outcome, precise quantification of periAR is of paramount importance but remains technically challenging.
METHODS:
The severity of periAR was prospectively evaluated in 146 patients treated with the Medtronic CoreValve (Minneapolis, Minnesota) prosthesis by echocardiography, angiography, and measurement of the aortic regurgitation (AR) index, which is calculated as ratio of the gradient between diastolic blood pressure (DBP) and left ventricular end-diastolic pressure (LVEDP) to systolic blood pressure (SBP): [(DBP – LVEDP)/SBP] × 100.
RESULTS:
After TAVI, 53 patients (36.3%) showed no signs of periAR and 71 patients (48.6%) showed only mild periAR, whereas 18 patients (12.3%) and 4 patients (2.7%) suffered from moderate and severe periAR, respectively. The AR index decreased stepwise from 31.7 ± 10.4 in patients without periAR, to 28.0 ± 8.5 with mild periAR, 19.6 ± 7.6 with moderate periAR, and 7.6 ± 2.6 with severe periAR (p < 0.001), respectively. Patients with AR index <25 had a significantly increased 1-year mortality risk compared with patients with AR index ≥25 (46.0% vs. 16.7%; p < 0.001). The AR index provided additional prognostic information beyond the echocardiographically assessed severity of periAR and independently predicted 1-year mortality (hazard ratio: 2.9, 95% confidence interval: 1.3 to 6.4; p = 0.009).
CONCLUSIONS:
The assessment of the AR index allows a precise judgment of periAR, independently predicts 1-year mortality after TAVI, and provides additional prognostic information that is complementary to the echocardiographically assessed severity of periAR.
Gotzmann M, Lindstaedt M, Mügge A. (2012). From pressure overload to volume overload: Aortic regurgitation after transcatheter aortic valve implantation. Am Heart J. 2012 Jun;163(6):903-11. [full abstract provided].
Severe aortic valve stenosis is a common valvular heart disease that is characterized by left ventricular (LV) pressure overload. A lasting effect of pressure overload is LV remodeling, accompanied by concentric hypertrophy and increased myocardial stiffness. Transcatheter aortic valve implantation (TAVI) has emerged as an alternative to surgical aortic valve replacement for patients with severe symptomatic aortic valve stenosis and high surgical risk. Although TAVI has favorable hemodynamic performance, aortic valve regurgitation (AR) is the most frequent complication because of the specific technique used for implantation of transcatheter valves.
During implantation, the calcified native valve is pushed aside, and the prosthesis usually achieves only an incomplete prosthesis apposition. As a consequence, the reported prevalence of moderate and severe AR after TAVI is 6% to 21%, which is considerably higher than that after a surgical valve replacement. Although mild AR probably has minor hemodynamic effects, even moderate AR might result in serious consequences. In moderate and severe AR after TAVI, a normal-sized LV with increased myocardial stiffness has been exposed to volume overload. Because the noncompliant LV is unable to raise end-diastolic volume, the end-diastolic pressure increases, and the forward stroke volume decreases. In recent years, an increasing number of patients have successfully undergone TAVI. Despite encouraging overall results, a substantial number of patients receive neither symptomatic nor prognostic benefits from TAVI. Aortic valve regurgitation has been considered a potential contributor to morbidity and mortality after TAVI. Therefore, various strategies and improvements in valve designs are mandatory to reduce the prevalence of AR after TAVI.
Walther T , Thielmann M, Kempfert J, Schroefel H, Wimmer-Greinecker G, Treede H, Wahlers T, Wendler O. (2012). PREVAIL TRANSAPICAL: multicentre trial of transcatheter aortic valve implantation using the newly designed bioprosthesis (SAPIEN-XT) and delivery system (ASCENDRA-II). Eur J Cardiothorac Surg. 2012 Aug;42(2):278-83. Epub 2012 Jan 30. [full abstract provided].
OBJECTIVE
Transapical (TA- aortic valve implantation (AVI) has evolved as an alternative procedure for high-risk patients. We evaluated the second-generation SAPIEN xt ™ prosthesis in a prospective multicentre clinical trial.
METHODS
A total of 150 patients (age : 81.6; 40.7 % female) were included. Prosthetic valves (diameter :23 mm (n= 36), 26 mm (n= 57) and 29 mm (n= 57) were implanted. The ASCENDRA-II™ modified delivery system was used in the smaller sizes. Mean logistic EuroSCORE was 24.3% and mean STS score was 7.5 ± 4.4%. All patients gave written informed consent.
RESULTS:
Off-pump AVI was performed using femoral arterial and venous access as a safety net. All but two patients receivec TA-AVI, as planned. The 29-mm valve showed similar function as the values of two other diameters did. Three patients (2%) required temporary bypass support.
Postoperative complications included renal failure requiring long-term dialysis in four, bleeding requiring re-thoracotomy in four, respiratory complication requiring re-intubation in eight and septsis in four patients, respectively.
Thirty day mortality was 13 ( 8.7%) for the total cohort and 2/57 (3.5%) receiving the 29 mm valve respectively. Echocardiography at discharge showed none or trivial incompetence (AI) in 71% and mild-AI in 22% of the patients. Post-implantation AI was predominantly para-valvular and > 2+ in 7% of patients. One patient required re-operation for AI within 30 days.
CONCLUSION
The PREVAIL TA multicenter trial demonstrates good functionality and good outcomes for TA-AVI, using the SAPIEN xt ™ and its second generation ASCENDRA-II™ delivery system, as well successful introduction of the 29-mm SAPIEN XT ™ valve for the benefit of high-risk elderly patients.
Subramanian S, Rastan AJ, Holzhey D, Haensig M, Kempfert J, Borger MA, Walther T, Mohr FW. (2012). Conventional Aortic Valve Replacement in Transcatheter Aortic Valve Implantation Candidates: A 5-Year Experience. Ann Thorac Surg. July 19 2012 [full abstract provided].
BACKGROUND:
Patient selection for transcatheter aortic valve implantation (TAVI) remains highly controversial. Some screened patients subsequently undergo conventional aortic valve replacement (AVR) because they are unsuitable TAVI candidates. This study examined the indications and outcomes for these patients, thereby determining the efficacy of the screening process.
METHODS:
Between January 2006 and December 2010, 79 consecutive patients (49% men), aged older than 75 years with high surgical risk, were screened for TAVI, but subsequently underwent conventional AVR through a partial or complete sternotomy. The indications, demographics, and outcomes of this cohort were studied.
RESULTS:
Mean age was 80.4 ± 3.6 years. Mean left ventricular ejection fraction was 0.55 ± 0.16, and the mean logistic European System for Cardiac Operative Risk Evaluation was 13% ± 7%. Of the 79 patients, 6 (7.6%) had prior cardiac surgical procedures. Indications for TAVI denial after patient evaluations were a large annulus in 31 (39%), acceptable risk profile for AVR in 24 (30%), need for urgent operation in 11 (14%), and concomitant cardiovascular pathology in 5 (6%). Mean cross-clamp time was 55 ± 14 minutes, and cardiopulmonary bypass time was 81 ± 21 minutes. Concomitant procedures included a Maze in 12 patients (15%). Postoperative morbidity included permanent stroke in 2 (2.5%), respiratory failure in 9 (11%), and pacemaker implantation in 2 (2.5%). Hospital mortality was 1.3% (1 of 79). Cumulative survival at 6, 12, and 36 months was 88.5%, 87.1% and 72.7%, respectively.
CONCLUSIONS:
Our existing patient evaluation process accurately defines an acceptable risk cohort for conventional AVR. The late mortality rate reflects the advanced age and comorbidities of this cohort. The data suggest that overzealous widening of TAVI inclusion criteria may be inappropriate.
Industry fights back
Now it looks like Edwards Lifesciences, the company that manufacturers the Sapien valve is speaking out to dispute recent findings that show TAVI to have less than optimal results. Of course, the author at the site, Med Latest says it best, “Setting aside the conflict of interest stuff, which might be a red-herring, what we’re left with is a situation where evidence-based medicine, while being something all would sign up to, is not that straightforward.”
[1] Several cardiologists and cardiac surgeons contributed to this article. However, given the current politics within cardiology, none of these experts were willing to risk their reputations by publically disputing the majority opinion. This is certainly understandable in today’s medico-legal climate in wake of widespread scandals and credibility issues. However, all quotes are accurate, even if unattributable with minor formatting (such as the addition of quotations, and paragraph headings have been added for increased clarity of reading in blog format.) I apologize for the ‘anonymous nature’ of my sources in this instance – however, I can assure you that these ‘experts’ know what they are talking about.
[All commentary by Cartagena Surgery are in italics and brackets].